![]()
(New tips are continually added to these pages. Check back in a few months' time for more)
TOPIC 14: Quantum Physics and Line Spectra
Tip 1:
What is the difference between Ionisation Energy and Work Function?
Ionisation Energy is the energy required to remove the outermost electron in an atom in its ground state to infinity, ie. cause the atom to become ionised.
Work Function, on the other hand, is the energy required to remove an electron from the surface of a metal to the vacuum level outside.
Obviously, Ionisation Energy is greater than Work Function.
Examples of Ionisation Energy vs. Work Function for some elements are presented in the table below:
|
Element |
Ionisation Energy (eV) |
Work Function (eV) |
|
Lithium |
4.34 |
2.17 |
|
Copper |
7.52 |
3.76 |
|
Silver |
8.68 |
4.34 |
Tip 2:
In the photoelectric equation:
KEmax = hf - Ø
KEmax refers to the Kinetic Energy of the most energetic photo-electron emitted from the metal surface.
Subsequently, when a reverse potential is created between the anode and cathode in the vacuum tube set up, the Stopping Potential (-Vs) refers the that reverse potential that stops even the most energetic electron (ie. those whose K.E. equal to KEmax ). Therefore,
eVs = KEmax
eVs = hf - Ø
Once this concept is understood, the photocurrent vs. potential graph becomes clear:
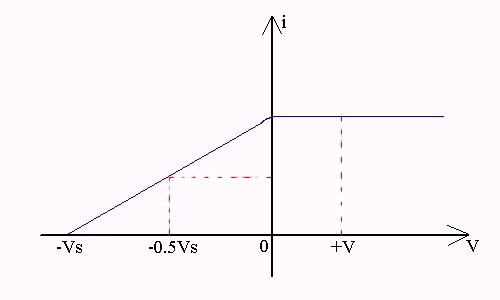
Referring to the above diagram, when the potential is positive (+V) (ie. anode higher potential than cathode), all photoelectrons, regardless of energy, will be attracted to the anode. Hence photocurrent is maximum.
When potential is -0.5Vs, those photoelectrons that have lower K.E.s will not be able to make it to the anode. Hence photocurrent decreases.
When potential is -Vs, even the most energetic photoelectron is repelled by the electric field. Hence no photocurrent.
© Copyright Physics.com.sg (Registration No. 52890077C). All rights reserved.
® First Class in Physics Tuition is the
Registered Trademark (TM No. T02/02149B) of Physics.com.sg
.